Technologies
Oyster PointMaking Dry Eye Easier to Treat:
An Interview with Michael Ackermann of Oyster Point Pharma
What is the need you set out to address?
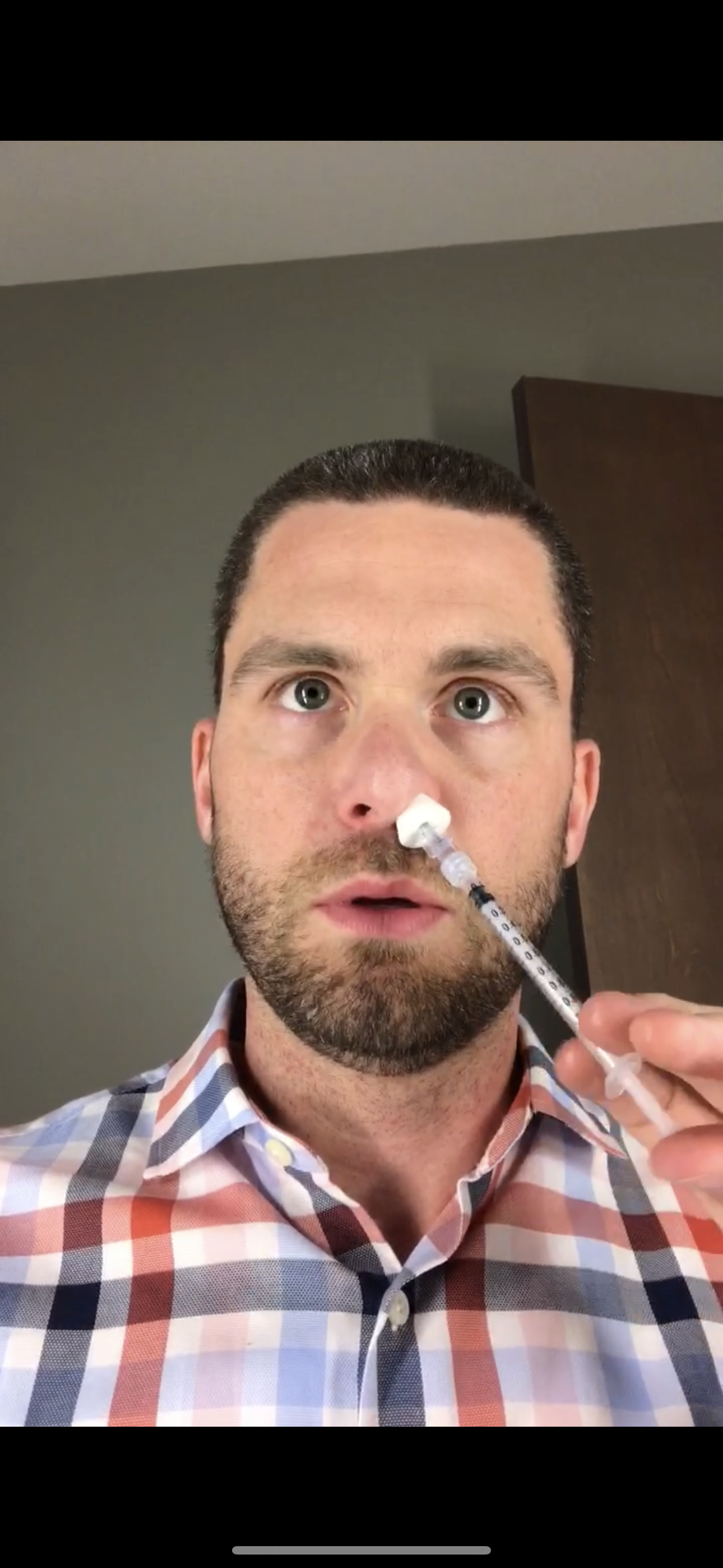
Michael: When I was an Innovation Fellow, one of our lead projects focused on addressing dry eye, which is an unmet need that affects roughly 30 million Americans. Symptoms of the disease range from discomfort and sensations of dryness to incapacitating pain and vision impairment. My team and I developed a neurostimulation solution that that we brought into patient care via a company called Oculeve [which was later acquired by Allergan]. Patients insert the tips of the device into their nasal cavity, which emit tiny pulses of energy that send nerve signals to the brain and then back to the tear glands to stimulate natural tear production. Most patients achieve results using the device 2-3 times a day for 60 seconds.
Even though the Oculeve device was efficacious, we realized that in order to provide a solution that a broad population can benefit from, it needed to be really easy to use. The device solution required charging, patients had to carry it around with them and the tips had to be changed regularly. For those with severe dry eye, ease of use is a nice-to-have criterion. But for those with mild to moderate disease, it’s a must-have. So, we asked ourselves what a competitor would do to make a solution that would be even easier for people to adopt and stick with.
"We asked ourselves what a competitor would do to make a solution that would be even easier for people to adopt and stick with."
What key insight was most important to guiding the design of your solution?
Michael: Since the Oculeve device works by stimulating tear production via the nasal passage, we wondered if we could develop an easy-to-use nasal spray to address the condition.
We dove deep into understanding the physiology of the nerves in the nasal cavity and whether they could be drugged to upregulate tear production. Then, through an extensive literature search, we identified 10 classes of receptors to screen. Our first goal was to determine if they would upregulate tear production; the second was to see if they would be comfortable. Only one class of drug was both effective and comfortable, and that became the basis of our solution.

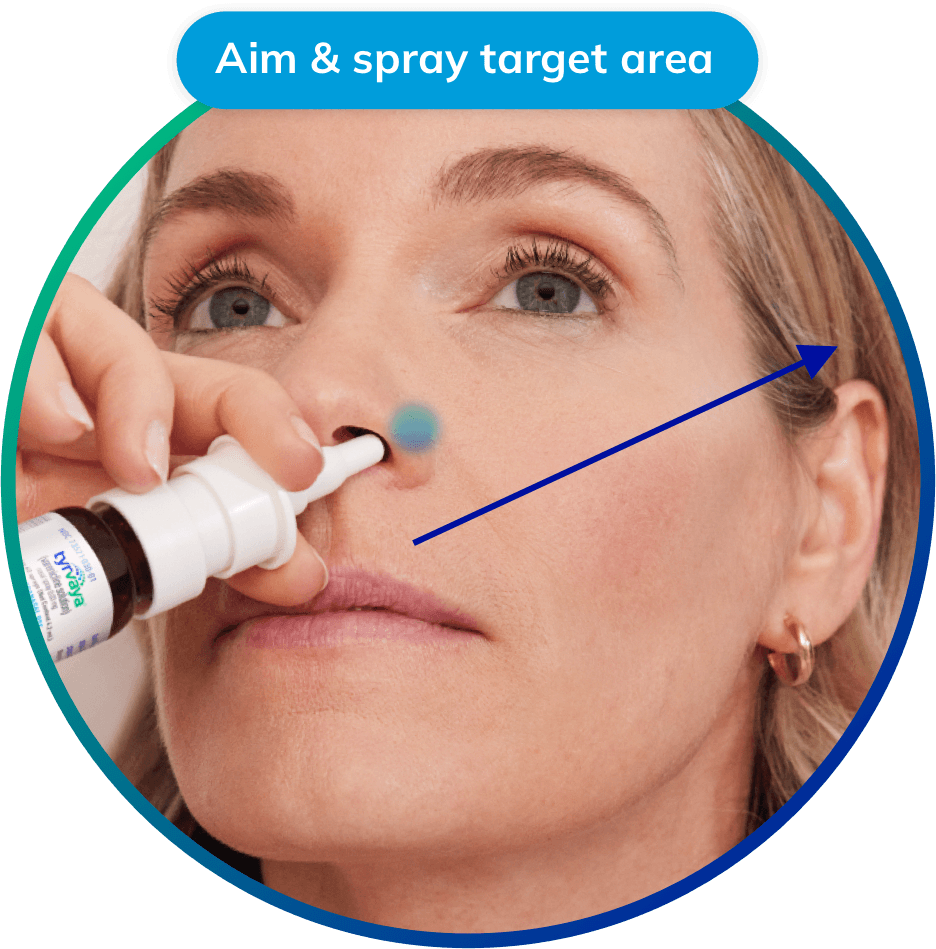
 The Oyster Point Pharma solution is a prescription nasal spray intended to treat the signs and symptoms of dry eye disease.
The Oyster Point Pharma solution is a prescription nasal spray intended to treat the signs and symptoms of dry eye disease.
How does your solution work?
Michael: Tyrvaya is a prescription nasal spray that treats the signs and symptoms of dry eye disease. Patients use it twice a day, 12 hours apart, to activate the body’s natural production of tears. The drug, varenicline, works as an agonist by binding to nicotinic acetylcholine receptors in the nerves that innvervate the nose. This binding activates the trigeminal parasympathetic pathway in thenose to increase basal tear production
At what stage of development is the solution?
Michael: The product has been commercially available since early 2022.
Tell us about a major obstacle you encountered and how you overcame it.
Michael: The big challenge for this need was determining if we could activate the target nerve pharmaceutically or not, and if we could, if we could do it in a way that didn’t create any uncomfortable sensation for the patient. There was no direct literature evidence that we could rely on, so we took our best guesses, ordered a bunch of molecules off of Sigma, and ran trial-and-error experiments.
"There was no direct literature evidence that we could rely on, so we took our best guesses, ordered a bunch of molecules … and ran trial-and-error experiments."
What advice do you have for other innovators about health technology innovation?
Michael: Even if you have a technology that addresses a previously unmet need, it’s unlikely that the entire need area goes away. Continue to think about the space like a competitor or new entrant would so that you don’t miss opportunities for ongoing innovation.
Michael Ackermann co-founded Oyster Point Pharma in 2017 after completing the Innovation Fellowship in 2010-11. For more information about the solution, visit the Tyrvaya website.
Disclaimer of Endorsement: All references to specific products, companies, or services, including links to external sites, are for educational purposes only and do not constitute or imply an endorsement by the Byers Center for Biodesign or Stanford University.