Technologies
OneBreath A More Affordable Ventilator:
An Interview with Matt Callaghan of OneBreath
What is the need that OneBreath seeks to address?
I got interested in disaster preparedness when avian flu was becoming a major concern. Research showed that besides the shortage of trained healthcare providers, one of the greatest challenges in responding would be providing enough mechanical ventilators to treat everyone in need. Traditional ventilators are expensive, require specialized training to use, and have to be regularly maintained, which makes stockpiling them for emergencies impractical. So in a pandemic or emergency situation, ventilators were the rate-limiting step—the most expensive, the most cumbersome, the most complicated, and the least adaptable equipment.
I initially started thinking about designing a better, lower-cost ventilator that could be used for disaster relief in pandemics and mass casualty events, but as my teammates and I got deeper in to the research, we realized that there was a major need for effective but more affordable ventilator equipment in resource-constrained settings across the globe.
There are three main types of ventilators. Full-featured ventilators used in state-of-the-art intensive care and emergency rooms settings cost $20,000-$50,000 and require a skilled operator as well as a reliable external power supply. Portable or transport ventilators are less expensive ($8,000-$15,000), but have a short battery life and aren’t approved for critical care patients. Finally, the most basic devices are low-cost resuscitators that cost approximately $500-$1,000 and are manually operated by a skilled doctor or nurse. Once we understood this landscape, we decided to focus on the need for an affordable ventilator capable of supporting patients in acute respiratory distress in low-resource environments with limited infrastructure and novice users.
What key insight was most important to guiding the design of your solution?
There’s a lot of market segregation in the ventilator space. Different equipment is required for different settings. But, after doing extensive primary research in India, we realized that what was really needed was a single device that could be used in multiple settings. We could make something portable enough to be used in the field, but not so portable that battery life would compromised, or the display would be too small for use in the ER or ICU. And these dual characteristics would benefit users in resource-constrained environments. For instance, even in the ICU, the power can cut out or the supply of compressed gas can run out and operators have to be able to switch seamlessly to battery power or manually-delivered gas. Or maybe a facility can only afford to purchase a limited amount of equipment, so they need something they can use in the hospital and also bring into the field in emergency situations.
“We realized that what was needed was a single device that could be used in multiple settings.”
This insight led us to develop a hybrid ICU/portable ventilator. Surprisingly, nobody else had really done that.
How does your solution work?
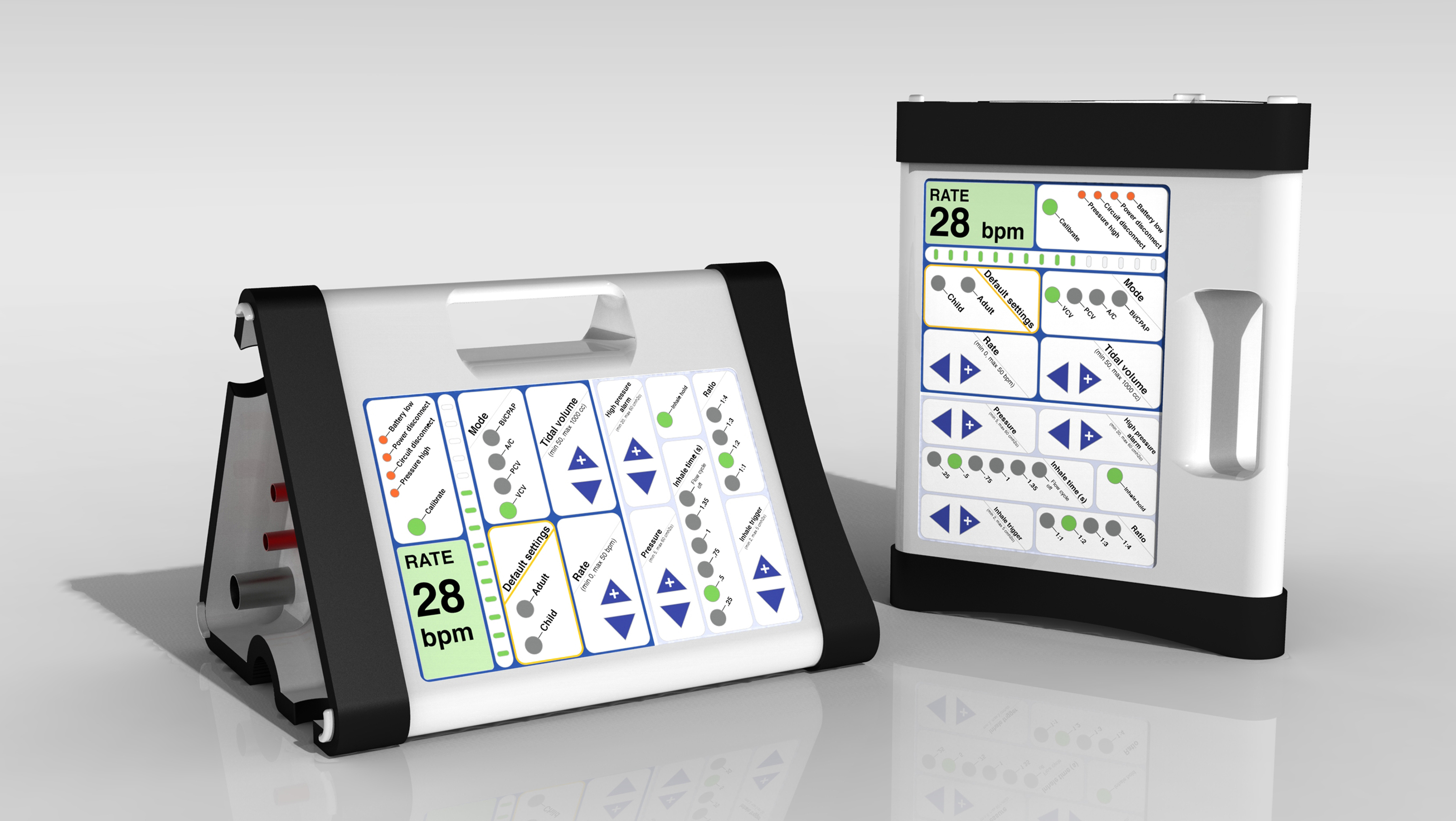
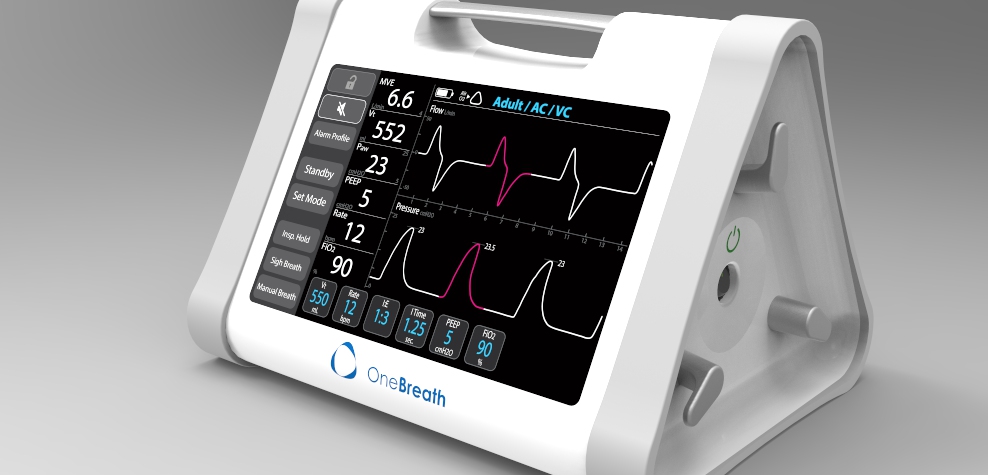
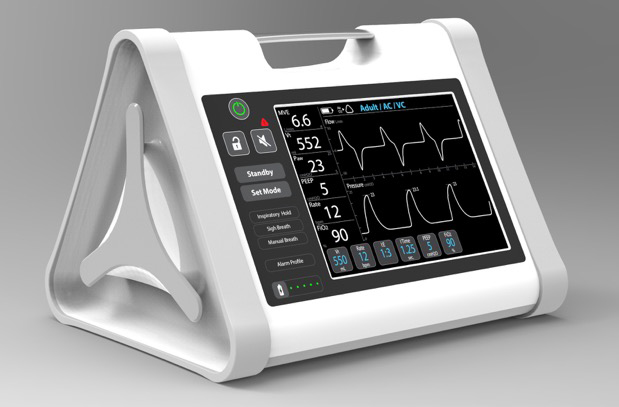
The OneBreath ventilator is a full‐featured ventilator intended for hospital and pre-hospital use in emerging markets. It’s optimized for low resource environments, novice users, and adult and pediatric patients. By starting completely from scratch, we were able to develop a simpler and less expensive design architecture, with a cost of goods that is a fraction of currently available ventilators but that provides standard-of-care performance and features. Our device is also comparatively easy to repair and requires relatively little maintenance. This is especially important for resource-constrained users working in demanding physical environments, where the maintenance and repair infrastructure is limited.
At what stage of development is the solution?
We’ve figured out manufacturing, our supply chain is locked in, all of our vendors have been audited, the hardware is complete, and the user interface is done. But we’re still working on refining and testing the software, which has been a challenge.
Once that’s done, we’ll seek regulatory clearance. Our plan is to pursue our CE mark in parallel with 510(k) clearance from the FDA because the requirements are harmonized.
Tell us about a major obstacle you encountered and how you overcame it.
Ventilators are capital equipment. They’re a commodity. You don’t need any “missionary” selling. Any physician who needs a ventilator knows what it is and how to use it. Ours just works better and costs less.
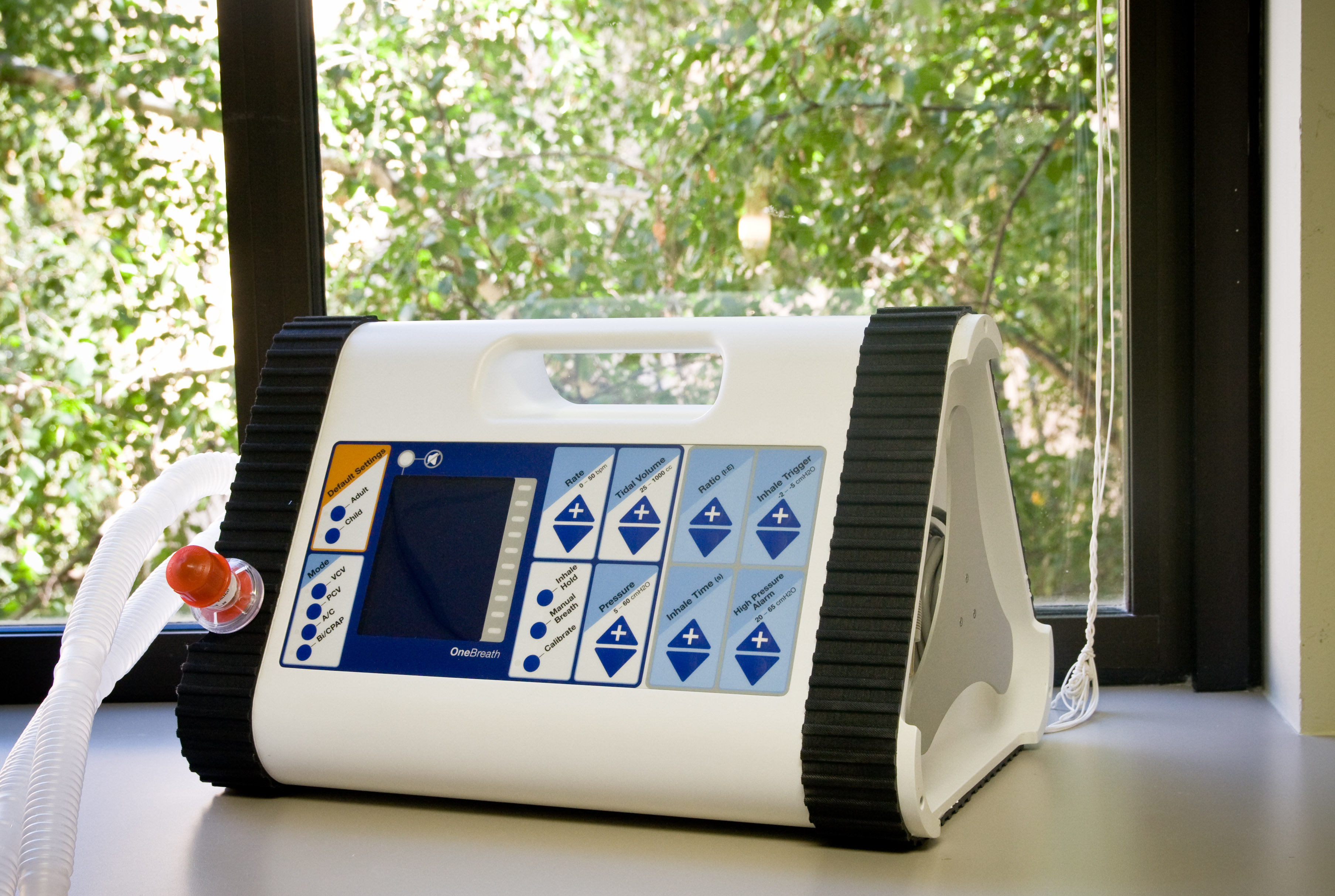
 OneBreath is an affordable ventilator designed to support patients in respiratory distress in low-resource environments.
OneBreath is an affordable ventilator designed to support patients in respiratory distress in low-resource environments.
You would think that would make the product easy to sell. However, in our target markets around the Global South, third-party distributors have a stranglehold on the hospital relationships. They take high margins and they’re not very transparent.
When we launch, we estimate that we can bring in about 25 percent of our target sales by selling directly to the hospital that have already been partnering with us in our pilot tests. But, at some point, we're going to have to put ourselves at the mercy of those third-party distributors.
 Matt Callaghan and the early OneBreath team.
Matt Callaghan and the early OneBreath team.
Figuring out how to do this most effectively has been difficult. I think the best way work with the distributors is not to approach them individually, but to partner with a strategic that already has good third-party channel. If the strategic doesn't have anything in its portfolio that competes with OneBreath, then I think it will be willing to take us on. And then hopefully the distributors’ loyalty to the strategic will help ensure that they give us a fair deal and represent the product well.
Ultimately, though, I don’t understand why we can’t just sell OneBreath online. People tell me that we can’t do this yet because it’s a critical care device. But there are a lot of rural hospitals that won’t have access to the product because even the distributors aren’t willing to ride a motorbike five hours outside Nairobi to sell three units. It's not worth it. But if a hospital could order our ventilator online and have it drop-shipped, we could reach the facilities that probably need OneBreath the most.
Reflecting on your experience, what advice do you have for other health technology innovators?
“Who would have thought that making a Honda would cost more than making a Ferrari?”
Making something inexpensive is a lot harder and more costly than making something expensive, if that makes any sense. Most of the time, to do a project like this, you would just buy parts from vendors you know you can rely on, regardless of cost. But, in our case, we needed to keep our cost of goods extremely low, so we had to value-engineer everything up front. For example, we couldn’t just go out and buy a touchscreen or a battery. We had to find someone who would make these components for us at a cost that made sense and negotiate with them to get it done. Then we had to get each component approved by our master manufacturer. So each step in the process takes months and months. Who would have thought that making a Honda would cost more than making a Ferrari? But it's true. There's just a lot of extra engineering that goes into ensuring that a product like this is cheap and reliable.
Matt Callaghan, founder and Chief Medical Officer of OneBreath, is a Biodesign Innovation Fellowship alumnus from 2008-09. To learn more, visit the OneBreath website.
Disclaimer of Endorsement: All references to specific products, companies, or services, including links to external sites, are for educational purposes only and do not constitute or imply an endorsement by the Byers Center for Biodesign or Stanford University.