Technologies
EarFlo Preventing Hearing Loss in Children:
An Interview with Intan Oldakowska and Matt Oldakowski of EarFlo
What is the need you set out to address?
Matt: Our focus is on treating children with otitis media with effusion in order to restore hearing while avoiding surgery. The population we're addressing is kids between the ages of one and five years old because 89% of kids with the disease are under five. It’s also the time when children are most affected by the condition because they're learning how to talk and socialize. There's strong evidence showing that if children don’t hear well in this age range, they quickly begin to fall behind and can experience significant developmental delays.
Intan: Otitis media with effusion is caused by the poor function of the eustachian tube, which is the canal that links the middle ear to the nasal-sinus cavity. The eustachian tube helps balance pressure and drain fluid from the middle ear. When it’s not working properly, fluid can build up behind the eardrum. When the eardrum can’t move freely, that causes hearing loss. Some children experience this for a few weeks, then the condition resolves on its own. Others have it for many months or years and require treatment. The most effective intervention currently involves surgery to place ear tubes to drain fluid and help balance pressure in the ear.
What key insight was most important to guiding the design of your solution?
Matt: One major insight was that it was such a simple mechanical problem. And this was one of the filters we used when screening needs: We were looking for simple mechanical problems that our mechanical minds could solve!
"We were looking for simple mechanical problems that our mechanical minds could solve!"
Our early research revealed some other important insights. There was a paper by a German physician who sat adult patients in a chair, angled their heads, and used a pneumatic otoscope to pressurize the eardrum and a politzer bag to pressurize the nasal cavity. He found that if he synchronized the three movements, he could clear 90% of effusions in adults. That helped convince us that the mechanism of action was sound. But when we started to investigate the devices that used this kind of pressure to open the eustachian tube and ventilate the middle air, we initially didn’t understand why they weren’t more broadly adopted. We bought them, started to play around with them, and read all the Amazon reviews. That’s when it became clearly apparent that they weren't practical at all, especially for children who are most impacted by the disease. With one device, you have to hold it to your nose, put some water in your mouth, and then press a button while you're swallowing. Even adult patients have problems coordinating that sequence of events. With another device, you have to blow up a balloon with your nose. Again, it’s very difficult, even for adults, to inflate the balloon and maintain the appropriate pressure without having air leak out. As a result, these devices really can’t be used for kids under five years old. The clinical trials show reasonably good data, but the average patient in these studies is age seven, which is outside critical one to five-year time frame. We realized that to effectively serve young children, we had to design something for a totally different user.
Intan: One other challenge with the current devices on the market is that the pressure is uncontrolled. So, it can be quite shocking for a young child when they’re able to coordinate their movements and use a device properly to clear the pressure in the ear. Many of them don't want to use it again because the sensation is so unpleasant.
How does your solution work?
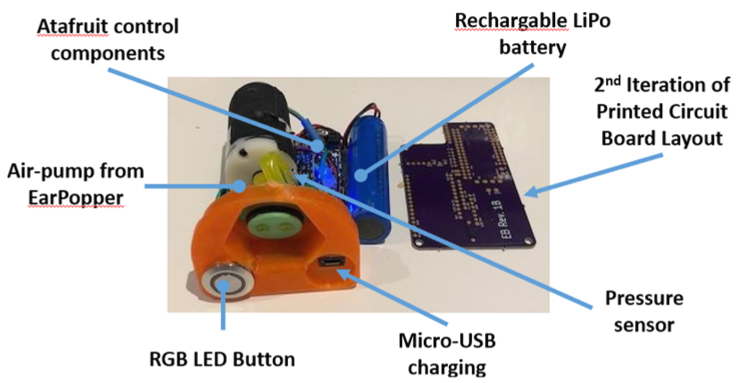
Matt: One thing children learn to do when they’re quite young is drink from a sippy cup. So, our solution leverages the basic form factor of a sippy cup, but with a built-in electric air pump to pressurize the nasal cavity when a child is naturally drinking. In order to get their lips on the sucker, the child has to press their nose into mask. That creates a seal so that when they swallow the fluid from the cup, their soft palate retracts to the back of the throat as the device initiates a brief and low-pressure puff of air that the child barely notices. But, because it’s precisely synchronized with the swallowing, it opens the eustachian tubes and ventilates the middle ear. That allows the fluid build-up to flow out of the middle air, which restores hearing. Importantly, the EarFlo device is sophisticated enough to make the process very simple for kids; just drink from the cup!
To help motivate children and make the experience fun, the device is paired with a game that can be played on a smartphone. In the game, they have a rocket that moves upwards based on intranasal pressure. When they get up to the right pressure, the rocket reaches a star. This helps them train themselves on how to use the device, and they get digital rewards each time they complete a session. Right now, each session requires them to get the desired effect five times. But we intend to study whether doing it more will cause children to get better even faster or whether five times is really necessary.
At what stage of development is the solution?
Intan: We’re at a clinical trial stage. We completed a single-use pilot study, using an early prototype, with good results. Thirty-one children, with an average age of about three years old, used the device once in a controlled environment and approximately 65% experienced a clinical improvement. We’ve now just started a pilot daily-use study with an injection-molded prototype that families can take home for four weeks. They'll use the device daily and we’ll check their diagnostic measures at the beginning, middle, and end of the study.
 Members of the EarFlo team: Peter Santa Maria, Steve Kargotich, Intan Oldakowska, and Matt Oldakowski.
Members of the EarFlo team: Peter Santa Maria, Steve Kargotich, Intan Oldakowska, and Matt Oldakowski.
 The EarFlo device is intended to treat children with otitis media with effusion to reduce the need for surgery and prevent hearing loss.
The EarFlo device is intended to treat children with otitis media with effusion to reduce the need for surgery and prevent hearing loss.
We also have a separate ethics approval to trial the device in Aboriginal children in the Australian Pilbara region. Otitis media with effusion affects Aboriginal children at roughly twice the rate of other children in Australia, which is the highest prevalence in the world. We’ve just started our single-use study in this community, with really exciting early results.
Tell us about a major obstacle you encountered and how you overcame it.
Matt: One of the biggest challenges we’ve faced is making the product easy to use for kids and parents to support high user compliance. This has been difficult because it’s really hard to get kids of this age to do anything, even something as simple as putting on a hat! Additionally, we had to make sure the device works with many different facial anatomies. As they grow, children’s facial anatomy changes. We also have to take into account how facial features may vary by ethnic group. So, it has been hard to create a one-size-fits-all mask.
Ultimately, we decided that we’d start with two different masks. The first is for kids in the three to five-year-old group that have somewhat more mature features and the physical dexterity to use the device by themselves. The second mask is for younger children who need help from an adult to use the device. There are different constraints with this design. For example, the adult has to be able to help the child position the mask, so we made the mask transparent so it’s easier for them to achieve proper alignment. It also has to be softer and more flexible so that it’s comfortable when fitting it around the young child’s nose. Getting these two designs right has required a lot of testing and iteration.
What role did your Biodesign training play in enabling you to design, develop, and/or implement this solution?
Matt: As the challenge above demonstrates, we’ve benefited a lot from deeply understanding the differing interests and requirements of the most relevant stakeholders and adopting empathy-based design principles. Staying focused on this through the project has been really influential.
"We’ve benefited a lot from deeply understanding the differing interests and requirements of the most relevant stakeholders and adopting empathy-based design principles."
Another important stakeholder example deals with the interests of the surgeons who normally treat otitis media with effusion. We initially were worried that they might resist a new solution in this space because it could reduce the number of surgeries they perform. But after going deep into our research, there are a couple of reasons why we don't think that's the case. First, we’re never going eliminate all surgeries. For instance, kids with cleft palate have this problem over and over again, very severely, so it probably makes sense for them to have ear tube surgeries. Second, we believe our device can help facilitate families moving more quickly into surgeries in cases where they really need them. Right now, surgeons spend a lot of time in clinic visits during the watchful waiting period, counseling families about the need for surgical intervention if the condition doesn’t resolve over a period of time because parents are so reticent to choose surgery for young children. If families try and fail our intervention, they’ll have a stronger reason why surgery may be necessary. And that can help make things more efficient for the surgeons and free up time to focus on more surgeries.
What advice do you have for other innovators about health technology innovation?
Matt: There are a lot of programs in Australia for medical innovation and we frequently get asked to mentor into those programs. The biggest problem I see with many teams is that they've identified a solution that they've fallen in love with, and they won't listen to anyone who says it's not great. They’re entirely focused on getting all the engineering done so they can bring their technology to patients. In these cases, our advice is always to take a step back and become more problem – or need – focused. That way, even if the technology fails, the team can pivot to a new solution. We also encourage them to be extremely picky about the problem they decide to solve because they are committing themselves to many years of hard work, so it makes sense to only work on unmet needs with a strong market pull.
Intan: There’s another thing I’ll mention. Once you start getting really deep into your need and your solution, there’s not one person who can give you all the right advice. You, as the innovator, quickly start to become the real expert in the space. Consultants and advisors who focus on regulatory or reimbursement can give you input based on the piece of the puzzle they understand. But you have to put that guidance into context with everything else you’ve learned, and then use all of that collective information to make the decision that’s best for the project. This means you may not always follow the exact advice of people who are really senior and experienced because they don’t understand the whole landscape. That can feel uncomfortable, especially at first, but you have to learn to trust yourself.
Intan Oldakowska and Matt Oldakowski co-founded EarFlo in 2018 with Jozef Bartunek and Peter Santa Maria out of the Global Faculty Training program.
Disclaimer of Endorsement: All references to specific products, companies, or services, including links to external sites, are for educational purposes only and do not constitute or imply an endorsement by the Byers Center for Biodesign or Stanford University.