Stories
Stanford Researchers Study the Timelines from FDA Authorization to Medicare Approval
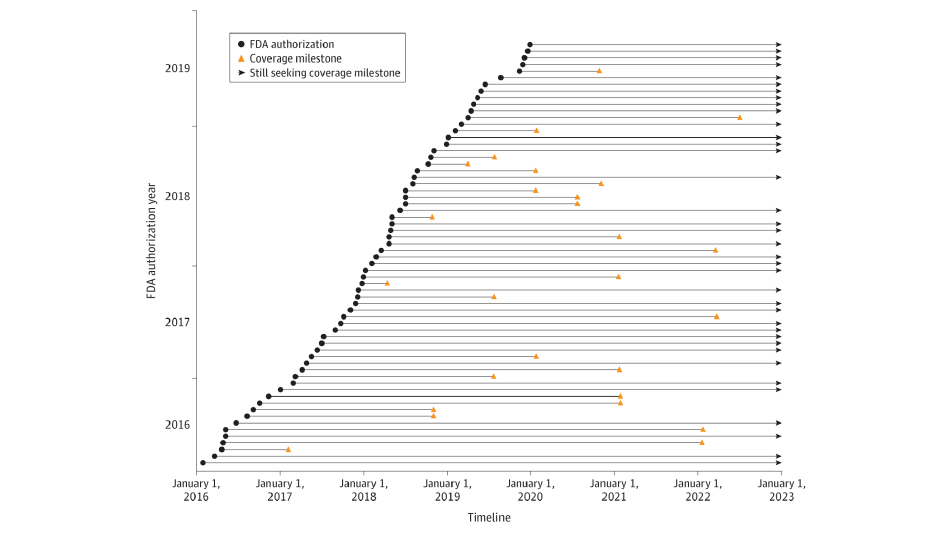 FDA indicates US Food and Drug Administration. Among 64 analyzed technologies that required establishment of new Medicare coverage, the time spent seeking a coverage milestone varied from less than 91 days to about 7 years.
FDA indicates US Food and Drug Administration. Among 64 analyzed technologies that required establishment of new Medicare coverage, the time spent seeking a coverage milestone varied from less than 91 days to about 7 years.
Every year physicians, researchers and other medical professionals create new technologies and diagnostic tools to improve patient care. And every year, the Food and Drug Administration evaluates these innovations to assure doctors and patients that the new tools and technologies are safe and effective. But that’s not the end of the story.
Even after the FDA authorizes novel technologies and diagnostic tools, the Centers for Medicare and Medicaid Services (CMS) must determine if these innovations are considered reasonable and necessary, the criteria for coverage by Medicare. In essence, new medical devices face a second review process in contrast to the implied coverage extended by CMS to new medications at the time of FDA approval. And because most private insurers follow Medicare’s lead, the decision by CMS can affect all patients who could benefit from new devices and diagnostics.
Stanford researchers wanted to better understand the timeline from FDA authorization to CMS coverage. The team studied the path of all novel medical technologies that were granted FDA authorization between 2016 and 2019 and were not already covered by Medicare. Published in the JAMA Health Forum, the research showed that of the 64 technologies that met the criteria, the majority, 56 percent of the innovations, were still waiting to achieve nominal coverage at the beginning of 2023.
“The median time to at least nominal coverage is 5.7 years,” said Zachary Sexton, a PhD candidate in Bioengineering who is the lead author on the study. “On average, this is longer than it takes to achieve FDA authorization. Furthermore, only a minority of technologies studied achieve this milestone suggesting that the average time is likely greater. CMS and members of Congress are looking at ways to change the process by which novel technologies achieve coverage and we believe this timeline data can inform new policy strategies.”
Policymakers have been grappling with ways to encourage novel technology development that is backed by data collection to prove the benefit of the technology in patient care. In January 2021, CMS published a rule called The Medicare Coverage of Innovative Technology and Definition of ‘Reasonable and Necessary’ (MCIT/R&N) that would have granted expedited Medicare coverage for up to four years, during which time device manufacturers could provide additional clinical data to achieve coverage. Nine months later, CMS repealed the rule.
This year, CMS published a procedural notice, Transitional Coverage for Emerging Technologies (TCET), that they stated was designed to deliver transparent, predictable, and expedited national coverage for eligible Breakthrough Devices with FDA authorization. Many stakeholders are concerned this rule does not go far enough to address the challenges many novel medical technologies face obtaining coverage by Medicare. In Congress, Rep. Brad R. Wenstrup (R-OH) introduced legislation to speed the delivery of groundbreaking medical technologies. The Ensuring Patient Access to Critical Breakthrough Products Act of 2023 would amend the Social Security Act to ensure prompt coverage of breakthrough devices in the Medicare program.
“Addressing the time to coverage for new technologies is important for Medicare beneficiaries, but also, per our prior published work, for the entire innovation ecosystem,” said Stanford Biodesign director Josh Makower, who is also an author on the study. “I am hopeful these data urge policymakers forward to advance a new and more robust pathway to coverage for novel and impactful medical technologies that have the potential to improve outcomes in areas of significant unmet clinical need.”
