Impact1
Translation
Bringing projects from an idea to patient bedside takes a team effort. Impact1 supports health technology innovators at all stages of development as they work on projects that improve the lives of mothers and children. Assistance ranges from offering project feedback to providing seed funding. In addition to working with external teams from universities, companies, or other external organizations, Impact1 has a pipeline of internal projects under development. Read on to learn more.
Project Support
We offer three levels of assistance for projects with a focus on pediatric or maternal patients – feedback, resources, and seed funding.
1. Feedback
Present your idea or project at the monthly Innovators Forum. Feedback is offered by experts in the field with diverse skillsets and backgrounds spanning medicine, engineering, finance, reimbursement, and more. Sign up here.
2. Resources
The most promising teams participating in the Innovators Forum are invited to receive further guidance and resources from Stanford Biodesign. Areas of frequent focus include assistance with need validation, market analysis, value analysis, regulatory strategy, reimbursement strategy, and preclinical/clinical study design. Depending on the stage of the project, directed resources can be made available to support specific project development needs.

3. Seed Funding
In 2018, UCSF and Stanford teamed up to launch the UCSF-Stanford Pediatric Device Consortium (PDC). The UCSF-Stanford PDC hosts an annual pitch competition for innovators developing pediatric medical devices.
The program offers awards of up to $100,000, thanks to the support of the FDA and the Cottrell Foundation. Winners also receive ongoing coaching and mentorship from the PDC. All US-based applicants across academia and industry are encouraged to apply. Learn more
Internal Product Development
The Impact1 team maintains a portfolio of early-stage health technology projects within Stanford University. Learn more about some of these projects below.
CatWatch: Umbilical Vein Catheter Migration Monitoring
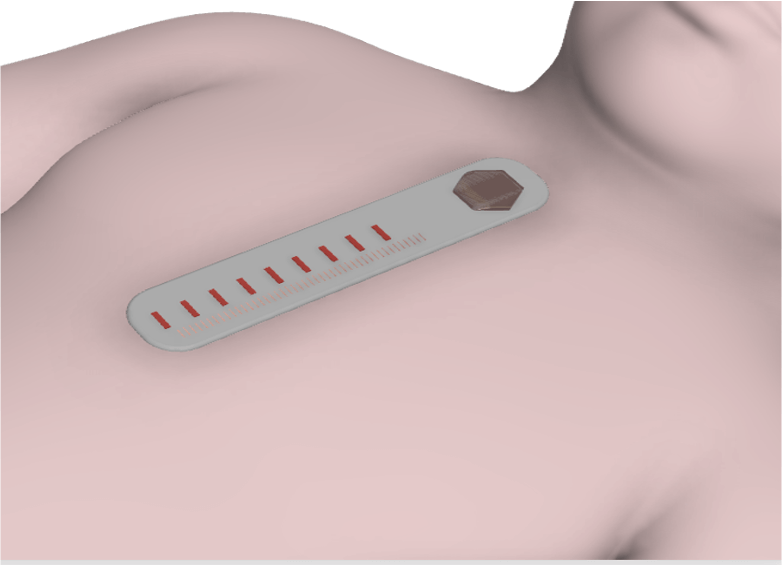
An umbilical vein catheter (UVC) is a life-saving technology for many premature infants. However, UVCs are often misplaced or migrate after placement, leading to complications and an increased cost of care. Currently, catheter placement assessment is performed only intermittently or in response to a change in clinical course. CatWatch fills the need for real-time, continuous bedside monitoring of UVC catheters in infants in order to prevent complications caused by central line migration.
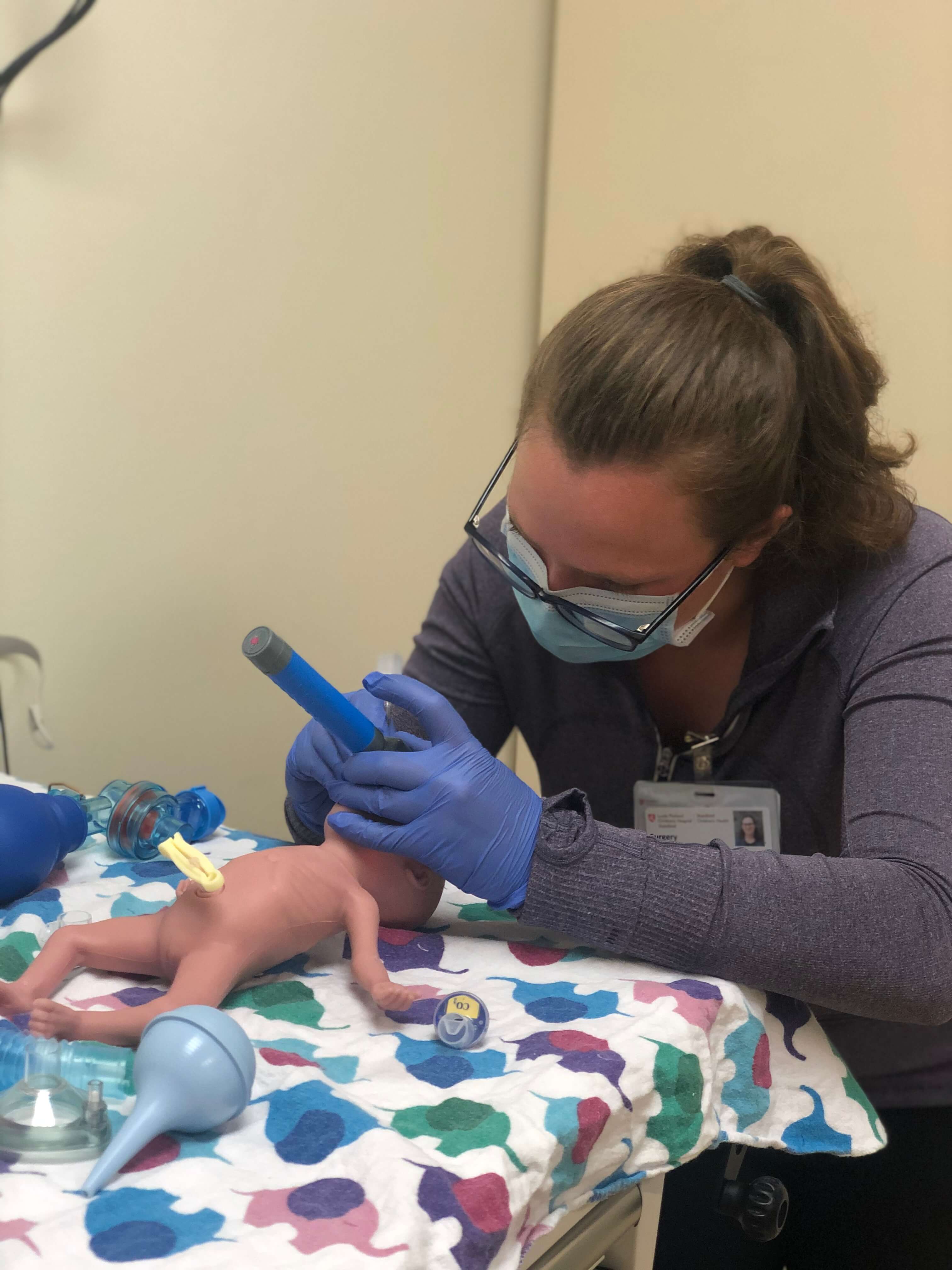
Entrepreneurship-in-Residence Pilot
A team of Stanford Biodesign innovators partnered with the Johnson Center for Pregnancy and Newborn Services at the Lucile Packard Children’s Hospital to address the biggest problems facing the smallest patients. The team began the six-month pilot program in December 2020 with interviews and observations in Stanford’s Lucile Packard Children’s Hospital. This clinical immersion led to the identification of 200 clinical problems that affect newborns or pregnant women. Following the biodesign process, the team selected two high impact solutions that could be carried through to product commercialization and patient use. We look forward to sharing more information as these projects develop.
The Entrepreneurship-in-Residence program has continued to be an internal initiative in which we develop pediatric and maternal health technology here at Stanford. The larger repository of needs that were identified in the pilot program will go on to serve as a pipeline for future translational development.
Research and Publications
The Impact1 team contributes to academic literature to promote the advancement of care for children and pregnant women. Publications can be found here.
Success Stories
The pediatric devices below have been conceived by Stanford faculty and students, and successfully launched as start-ups.

Novonate: Umbilical Catheter Securement and Protection
Biodesign-originated Novonate developed its first technology within Stanford. Umbilical catheterization is a lifeline for delivering medication and nutrition to critically-ill newborns in the neonatal intensive care unit. Read more
Eclipse Regenesis: Mechanical Treatment for Short Bowel Syndrome
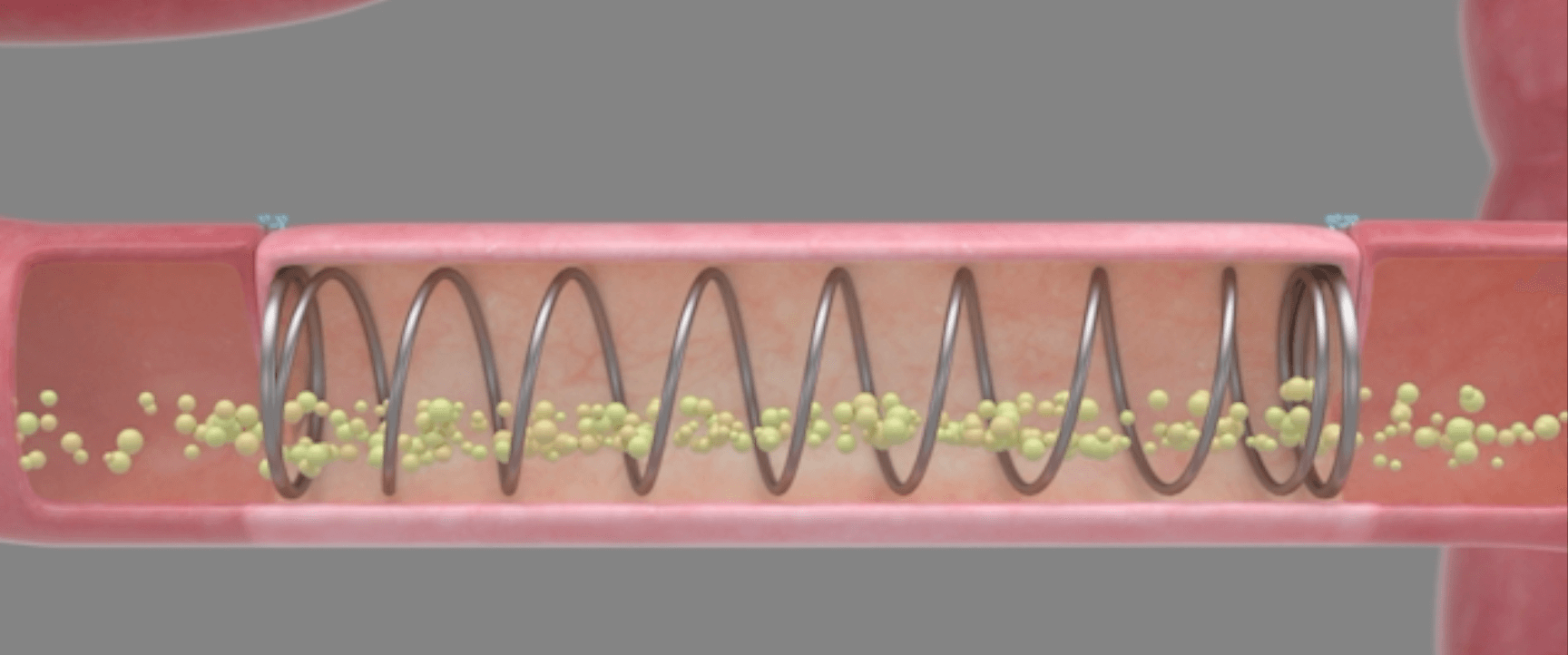
Eclipse Regenesis is developing a solution to short bowel syndrome in the form of tissue regeneration therapy. The device, named Eclipse XL1, is a mechanical approach designed to grow healthy intestinal tissue 2-3x in length within 2-3 weeks. Read more.
COVID Care Innovation
As COVID-19 cases started to escalate in the United States, PHTP and the UCSF-Stanford PDC supported innovators working to rapidly address needs created or exacerbated by the pandemic. Seed funding was granted to several Stanford-affiliated projects, ensuring that pediatric care was incorporated into the solutions from early design through implementation. Read about two of the projects.
