Technologies
BioTrace Rethinking Temporary Pacing Leads:
An Interview with Aravind Swaminathan of BioTrace Medical
What is the need BioTrace seeks to address?
Our clinical immersion in the Biodesign Innovation Fellowship was focused on cardiology. During our observations, we were surprised by the relatively high complication rates associated with the temporary pacing leads used for multiple cardiac procedures. Temporary pacing leads prevent the patient’s heart rate from slowing to dangerously low levels during certain procedures. Yet despite some of the complications associated with using them, such as cardiac perforation that can be fatal to patients, the technology hadn’t changed in nearly 40 years.
"We knew there had to be a safer, more effective way to provide temporary pacing."
With the anticipated rise of new procedures such as transcatheter aortic valve replacement [TAVR] that require the use of temporary pacing leads, we could see that there would be increasing dependence on this outdated technology. And that didn’t make sense. We knew there had to be a safer, more effective way to provide temporary pacing.
What key insight was most important to guiding the design of your solution?
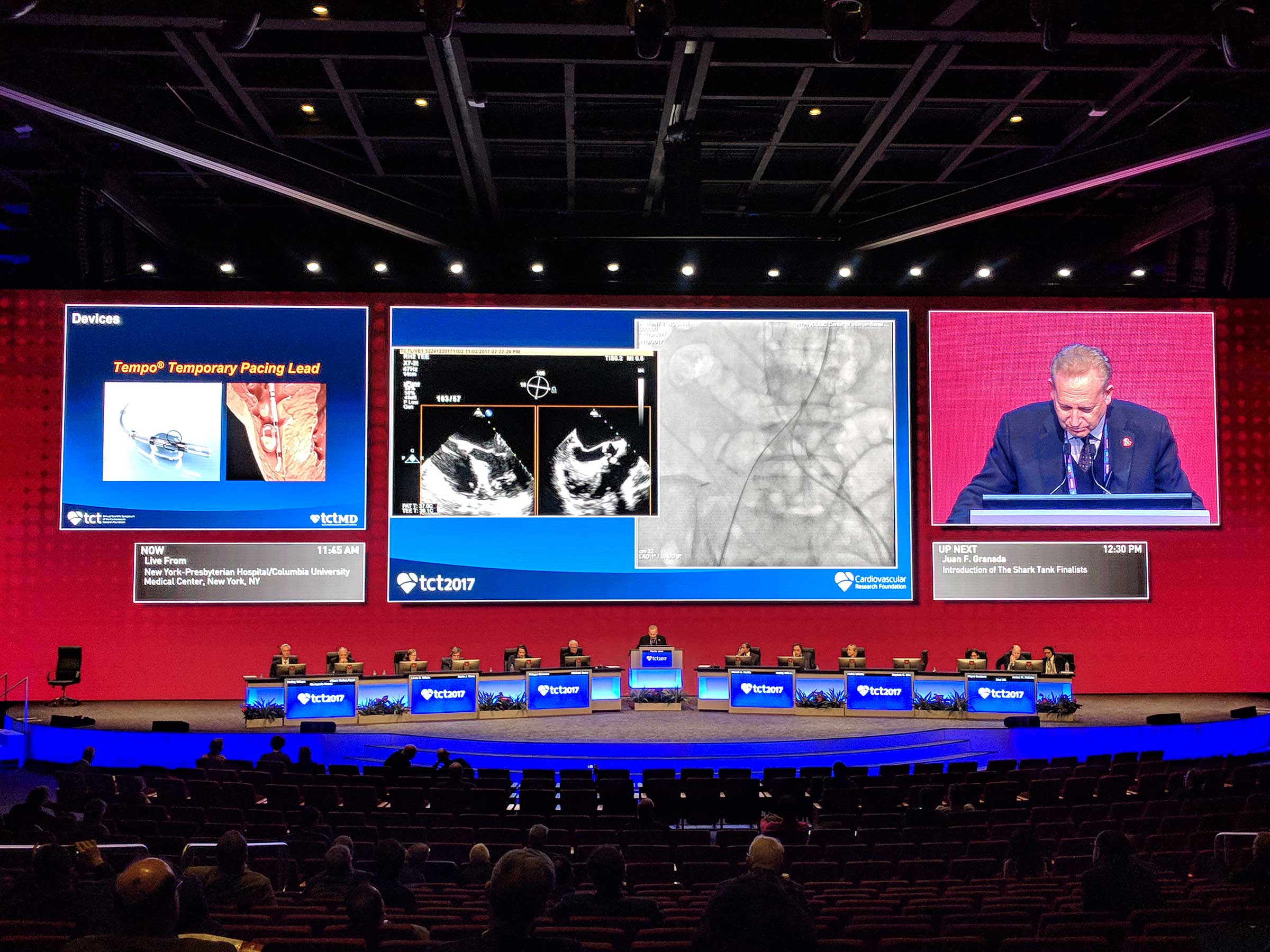
Most temporary pacing leads are catheter-based devices with a pair of electrodes on the distal end and a rigid metal tip that can be advanced into the heart. The leads are positioned in the right ventricle, but aren’t secured in place. This design creates a number of risks. The firm tip can be traumatic to the heart tissue and, in some cases, can result in cardiac perforation. And because the leads are not secured, dislodgment occurs in 10-40% of all cases.
Our technical insight was to move the rigid electrodes away from the tip of the device and use a soft, bendable material to reduce the risk of perforation. In doing so, we had to create a means to keep the pacing electrodes in contact with tissue. So, we also created a novel side-anchoring mechanism that deploys two stabilizer loops from within the lead into cardiac tissue to reduce the rate of dislodgment.
 Members of the BioTrace team with investigators from their first-in-human study: Ron Hundertmark (VP, R&D), Mark Webster (Auckland City Hospital, NZ) Aravind Swaminathan (CMO), Laura Dietch (President & CEO), Sanjeevan Pasupati (Waikato Hospital, NZ), and Evan VandenBrink (R&D Manager).
Members of the BioTrace team with investigators from their first-in-human study: Ron Hundertmark (VP, R&D), Mark Webster (Auckland City Hospital, NZ) Aravind Swaminathan (CMO), Laura Dietch (President & CEO), Sanjeevan Pasupati (Waikato Hospital, NZ), and Evan VandenBrink (R&D Manager).
We also had an important clinical insight. Because temporary pacing leads are used by many different kinds of physicians – cardiologists, surgeons, anesthesiologists, emergency room doctors, intensivists – we wanted to create a technology that could be implemented by all of them. That really informed the design of the device. Our goal was to develop something that was literally a snap to deploy.
How does your solution work?
The device, called the Tempo Lead, is floated into the heart through a catheter in the neck or groin. The soft tip enables it to land gently in the right ventricle, then a balloon is inflated to carefully wedge the lead in place. The stabilizing loops, which reside inside the lead, are deployed into the heart muscle and the balloon is deflated. Finally, the lead is hooked up to an external generator for cardiac pacing and pace capture. When temporary pacing is no longer needed, the physician uses the handle to retract the loops back into the lead so that it can be easily removed.
 The BioTrace Medical Tempo Lead is intended to provide secure and stable temporary cardiac pacing.
The BioTrace Medical Tempo Lead is intended to provide secure and stable temporary cardiac pacing.
The goal of this new approach is to minimize complications and allow patients to ambulate sooner after their procedure. We have been fortunate to perform and present a retrospective clinical study in the US that has shown our new Tempo Lead to be successful in this regard.
"The goal of this new approach is to minimize complications and allow patients to ambulate sooner after their procedure."
At what stage of development is the solution?
We received our FDA clearance in October 2016 and we initiated a limited commercial launch in early 2017. The reception been encouraging so far, with cardiologists and surgeons calling the technology “an exciting advance over existing technologies” and “the new standard of care for patients who need temporary pacemaker support.”
Our next steps will be to expand our commercial presence to make the technology more widely available and to look to clinical applications beyond TAVR.
Tell us about a major obstacle you encountered and how you overcame it.
As a small company, our biggest obstacle has been thinking about how to commercialize the technology so that patients everywhere can benefit from it. Building a sales force is time consuming and expensive. And when you’re a start-up, you have to make a name for yourself by gathering compelling clinical data and establishing a strong reputation. Once the device was cleared, our initial strategy was to approach the people who had helped and advised us to become early adopters of the technology. We also put out a press release, which got a number of people interested in using the solution. That approach helped us start building a track record.
What advice do you have for other innovators about health technology innovation?
Try not to have too much of an ego when it comes to the leadership of your project. Rather than attempting to run the company ourselves, we brought in an experienced CEO – Laura Dietch. She was able to recruit a strong VP of R&D and put together a seasoned team. This de-risked the project quite a bit, and I think our investors really appreciated it. It’s also a great way to learn as a first-time entrepreneur.
Aravind Swaminathan co-founded BioTrace Medical with Ellis Garai out of the Biodesign Fellowship in 2010. Laura Dietch joined as co-founder and CEO shortly thereafter. To learn more, visit the BioTrace Medical website.
Disclaimer of Endorsement: All references to specific products, companies, or services, including links to external sites, are for educational purposes only and do not constitute or imply an endorsement by the Byers Center for Biodesign or Stanford University.