Stories
It Takes a University: How a Determined Team Developed a Technology to Protect Vulnerable Newborns
 The Novonate Team: Venook, Chehab, and Wall.
The Novonate Team: Venook, Chehab, and Wall.
While in the project-based Stanford Biodesign Innovation course, which brings together graduate students in engineering, medicine, and business to address real world healthcare problems, one student team discovered that pediatric patients are five times more likely than adult patients to get catheter-related bloodstream infections. Umbilical cord catheters, which are used to provide food and medication to fragile, low birthweight newborns in the NICU, are particularly problematic.
“Although only about 200,000 newborns need umbilical catheters every year [in the US], one in five acquires a costly and dangerous bloodstream infection,” said team member Eric Chehab, who was a graduate bioengineering student at the time. [i]
“And this is despite the fact that the nurses watch over these babies really carefully because they are so vulnerable.” According to the team’s research, infants who have had these infections face longer hospital stays, an increased risk of developmental delays, and even death. [ii,iii]
Despite the relatively small number of babies affected, the team found it compelling that consequences were so serious and that the infections were largely preventable. They committed to inventing a solution. As they dug into the research, they quickly focused on the mechanism used to hold the catheters in place. “Unlike the sleek, sterile devices that protect and support central lines in adults, the nurses have to adapt bandages and tape. At Stanford they make a little bridge out of surgical tape to hold the catheter upright in the umbilical cord stump,” said Chehab.
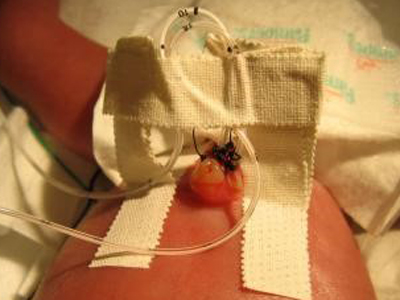 An example of how surgical tape is used to hold an umbilical catheter in place (photo from eMedicine.Medscape.com).
An example of how surgical tape is used to hold an umbilical catheter in place (photo from eMedicine.Medscape.com).
The team set out to design a device that would reduce the likelihood of infection by better supporting and protecting the umbilical line. Over the course of the next two quarters, they worked to address design challenges that included keeping the insertion site open to the air to permit desiccation of the stump, and supporting a catheter rising from the body perpendicularly rather than lying flat against the skin as an adult catheter would. By the end of the class, they had developed an initial prototype; a small, semi-rigid, open dome that could be secured against the baby’s skin.
Taking the Solution Forward
Though this early concept was promising, the possibility of taking it forward into patient care seemed remote. First, with the class over, most of the original team moved on to focus on other academic priorities. Second, and even more daunting, the market for the device was too small to attract traditional venture capital funding to advance the project. However, team advisor, James Wall, MD, a pediatric surgeon and assistant director of the Stanford Biodesign Innovation Fellowship, had an idea. “As a pediatric specialist, the need really resonated with me. And I thought that maybe we could maximize the resources inside Stanford University to develop this project as a technology with high impact but not necessarily a large market value.”
Wall brought in Ross Venook, assistant director of engineering for Stanford Biodesign and lecturer in bioengineering, and the two began to actively guide the project, now named Novonate, forward. They expanded the team include bioengineering lecturer Joe Shih, medical student Lauren Wood, and several bioengineering students, including Shivani Torres (BS in BIOE, 2015; MS in ME, expected 2017), Varun Jain (MS in BIOE, expected 2017), and Abhay Ramachandra (PhD in BIOE, 2016). Biodesign Innovation Fellowship alums Eric Kramer and Janene Fuerch, MD, lecturer Marlo Kohn, and Bill Rhine, MD, also joined as advisors.
With support from the MedScholars Research program and a Coulter Foundation Translational Research grant, the team developed and tested a series of prototypes to experiment with different form factors and materials. They also initiated biologic testing to measure protection from bacterial contamination, as well as mechanical testing to assess the performance of the design. “The fundamental need was to reduce infection rates, but it would take a long time and lot of money to run the studies we’d need to prove this to the FDA when seeking regulatory clearance,” said Wall. “A more stable catheter is less likely to get infected, [iv] so we focused on that."
Added Venook, “Specifically, we sought to stabilize the catheter to prevent pistoning, or vertical movement of the line that can introduce contamination into the vessel. We also wanted to isolate the site sufficiently to prevent the migration of bacteria from elsewhere on the skin, and the transfer of any bacteria from the touch of caregivers.”
The team also focused on developing and secure Stanford-owned intellectual property on the technology, and investigating regulatory options to better understand the path the device would have to take to get clearance from the FDA.
The deep resources at Stanford helped the Novonate team progress. “We started our benchtop research with goat umbilical cords,” said Wall. “Then, taking advantage of the fact that the engineering buildings are right near the hospital, we were able to get human umbilical cords from the obstetrics department.”
“Once we had positive bacterial and mechanical test results, we started to really focus on the user,” noted Venook. Referring to the umbilical cord taping that was currently the standard of care, he continued, “We felt that by replacing the need for this kind of ‘arts and crafts project,’ we could offer the NICU nurses value in terms of convenience, standardization, and ease of use, while hopefully reducing the most significant causes of infection.”
To find out what it would take to get the nurses excited about this new approach, the team returned to the Lucille Packard Children’s Hospital to meet with NICU nurses. Later, they traveled to conferences and sought input from other partner institutions, including the Children’s Hospital of Los Angeles and UCSF-Benioff Children’s Hospital (among others). “We spent a lot of time talking to nurses, neonatologists, buyers, and hospital administrators,” said Chehab, who at this point had completed his PhD in bioengineering, and re-engaged full-time with Novonate as its CEO.
By learning about the unique needs of the NICU nurses and the neonates they care for, the team was able to translate key insights into product features, such as making one side of the device red and the other blue to make it easy to distinguish an arterial line from a venous line. Additionally, noted Chehab, “With some of the early prototypes, the nurses still wanted to tape the catheter to the device, even though it wasn’t necessary. So we refined the design to make an audible click when the device is secure.”
The team presented the technology at the National Capital Consortium for Pediatric Device Innovation, an annual pediatric device meeting in Washington DC, as well as “Impact Pediatric Health,” an association of hospitals including Stanford that seeks to accelerate pediatric innovation through competition. Although the national exposure and positive feedback they received was encouraging, Novonate still needed to find an unconventional financial path forward. The answer came when the team was accepted into StartX, a Stanford-associated non-profit business incubator. At this point, the University agreed to co-invest in the project as well. With this funding, along with an SBIR grant from the National Science Foundation, Novonate was able to develop and de-risk the project sufficiently that it attracted additional investment from Medtech Venture Partners and a private angel investor.
“It took more than five years and the efforts of a lot of dedicated people to make this solution viable,” said Wall. “It is an incredibly important unmet need – literally saving premature babies. By utilizing a full range of university resources including students, facilities, and internal grants, we were able to develop the device to the point that it could attract funding – which would never have happened using traditional methods – and it is now being evaluated at multiple NICUs including Stanford and Oregon Health Sciences University.”
[i] All quotations are from interviews conducted by the authors unless otherwise cited.
[ii] Dubbink-Verheij GH, Bekker V, Pelsma IC, et al., "Bloodstream Infection Incidences of Different Central Venous Catheters in Neonates: A Descriptive Cohort Study," Frontiers in Pediatrics, June 20, 2017.
[iii] Bakhuizen S, de Haan TR, Tuene MJ, et al., "Meta-Analysis Shows that Infants Who Have Suffered Neonatal Sepsis Face an Increased Risk of Mortality and Severe Complications," Acta Paediatrica, September 7, 2014, http://onlinelibrary.wiley.com/doi/10.1111/apa.12764/full (December 20, 2017).
[iv] MacDonald MG, Ramasethu J, "Umbilical Artery Catheterization," Atlas of Procedures in Neonatology, 3rd edition, Philadelphia: Lippincott Williams & Wilkins; 2002.
