Technologies
Miret Less Invasive Surgical Tools:
An Interview with Avi Roop of Miret Surgical
As a Biodesign Innovation Fellow, what was the need you set out to address?
It was a way to further reduce the invasiveness of minimally-invasive surgery that preserved speed, cost, and safety.
What key insight was most important to guiding the design of your solution?
Laparoscopic or port-based surgery is commonly referred to as minimally-invasive surgery. During the fellowship, there was a lot of interest in reducing that invasiveness even further, and people were experimenting with new ways to accomplish that such as using a single trocar or operating through natural orifices. While these procedures were intriguing, most were failing for important reasons that reflect surgical realities. First and foremost, they took more than twice as long to perform, which made them expensive due to the cost of surgical time. In addition, the tools required to perform them were very expensive for the hospitals to purchase. Finally, to use these new techniques surgeons had to learn new ways to do things that they already knew how to do very well. So that made the new operative approaches higher risk.
So we realized that the only way to reduce the invasiveness of surgery effectively was to keep procedure time, safety, and cost constant. Those were the core requirements that came out of our need specification.
“We realized that the only way to reduce the invasiveness of surgery effectively was to keep procedure time, safety, and cost constant.”
How does your solution work?
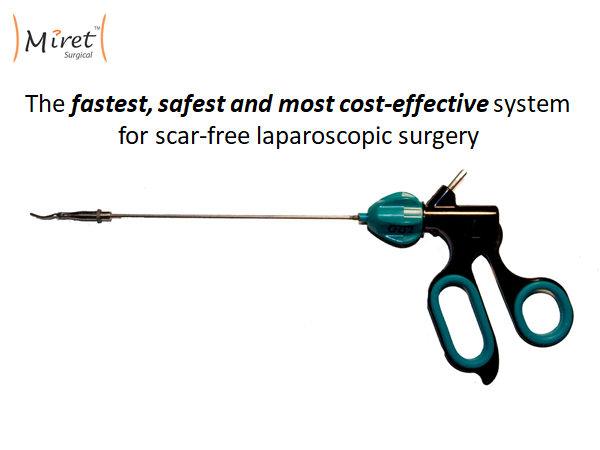
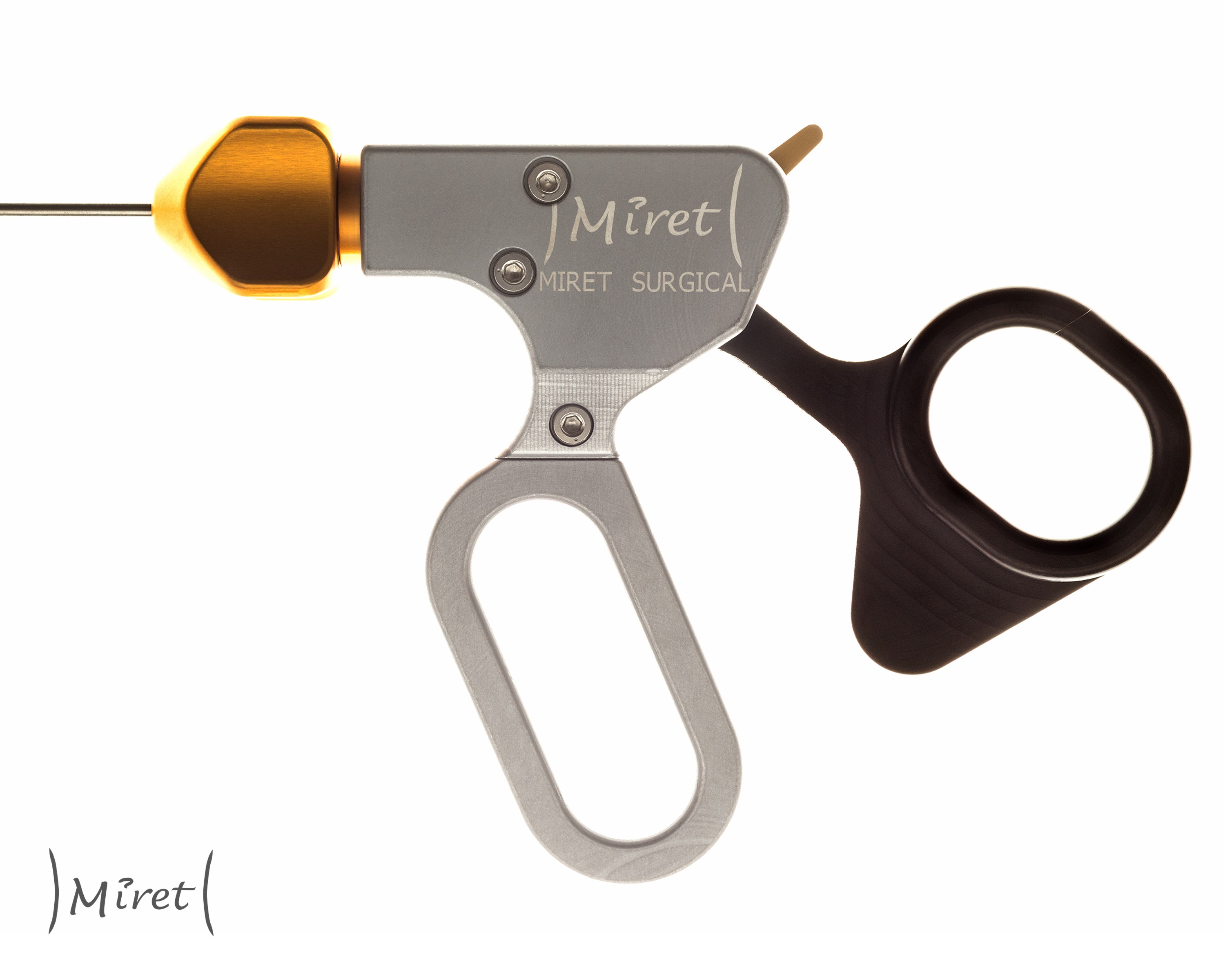
The Miret Surgical Percutaneous Surgical System reduces invasiveness by getting rid of the trocar and shrinking the diameter of the shaft down in order to reduce scarring. Currently, the surgical tool plus the trocar adds up to a 7-8mm diameter shaft. We shrink that down to 2.4mm. The trick is we don't force surgeons to use a tiny jaw head at the end of the 2.4mm shaft. We have developed a way to put standard-sized tool heads onto this percutaneous shaft inside the body. The only compromise we're asking surgeons to accept is dealing with this smaller diameter shaft across the abdominal wall. Everything else about the tool is the same. The placement is the same, the procedure is the same, and the cost is the same.
 The early Miret team, including Biodesign Innovation Fellows Avi Roop (far right) and Kevin Chao (front center).
The early Miret team, including Biodesign Innovation Fellows Avi Roop (far right) and Kevin Chao (front center).
 This short promotional video, provided by Miret, illustrates how the company's surgical system is intended to work (see endorsement disclaimer below).
This short promotional video, provided by Miret, illustrates how the company's surgical system is intended to work (see endorsement disclaimer below).
At what stage of development is the solution?
We've got FDA clearance, we've done some early cases, and we're currently exploring strategic partnerships.
Tell us about a major obstacle you encountered and how you overcame it.
I didn't really know what the market size for our technology was going to be. There are plenty of surgical cases, but each individual market [general surgery, thoracic surgery, etc.] has boundary conditions that could limit our opportunity. Alternatively, we could be viewed as a disruptive technology, which could lead to a much larger market opportunity. I didn't really know which way this would go, so I decided to pursue a lean strategy.
“I didn't really know what the market size for our technology was going to be… so I decided to pursue a lean strategy.”
The hurdle associated with this was that we were always asking for too little money. Our funding plan was well below even small-end venture capital investment sizes. I didn't realize that this mismatch would create such a huge impediment to raising investment in the company. So I ended up going with a nearly 100 percent angel strategy, which took a lot more time and effort.
The upside of that experience is now I have a core group of angel investors who have invested in Miret, and who have invested in my other projects, and will hopefully work with me for a long time. With that group of individuals, I'm able to raise most of the capital I need to do projects in the fashion that I like doing them, which is with a super capital-efficient approach. Some people call it ‘small ball’ but it's quite specifically trying to get products into the market for under $2 million.
Reflecting on your experience, what advice do you have for other health technology innovators?
Product development is hard. In any project, even if it seems straightforward on the surface, there's always something significant and unexpected that you have to learn how to do from the ground up. My advice is to be mentally prepared for this so that when it happens it doesn't feel devastating. You need to protect yourself by maintaining a certain amount of money in the bank and the emotional bandwidth to tackle these things as they arise.
Miret has taken longer than I initially thought it would. But I've learned to keep some spare gas in the tank so I can keep going when pushes are required.
Avi Roop is the CEO and co-founder of Miret Surgical. He launched the company from the Biodesign Innovation Fellowship in 2009 with Biodesign Innovation Fellows Kevin Chao and a team of others. To learn more, visit the Miret Surgical website.
Disclaimer of Endorsement: All references to specific products, companies, or services, including links to external sites, are for educational purposes only and do not constitute or imply an endorsement by the Byers Center for Biodesign or Stanford University.